ACIDS, BASES AND SALTS
11/1/20243 min read
Acids and Bases
Acid:
An acid is a substance that:
Tastes sour.
Can conduct electricity.
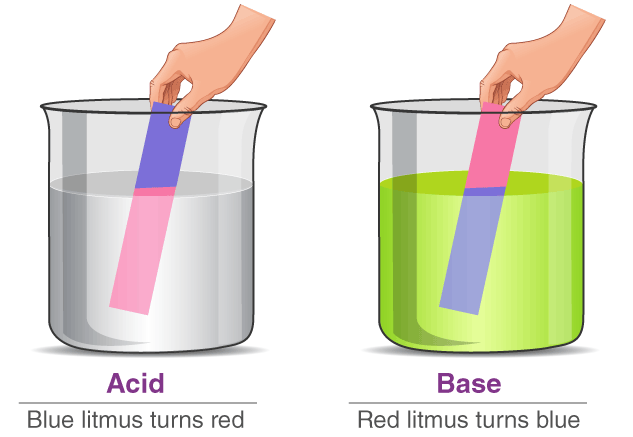
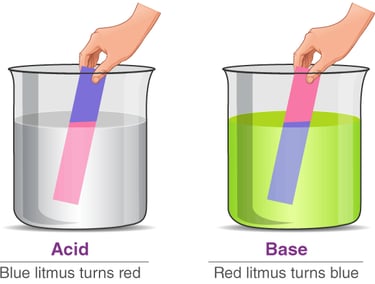
Turns blue litmus paper red.
Releases hydrogen ions (H⁺) when dissolved in water.
Has a pH level below 7.
Examples: Curd, lemon juice, orange juice, vinegar etc.
Base:
A base is a substance that:
Tastes bitter.
Feels slippery.
Turns red litmus paper blue.
Accepts hydrogen ions (H⁺) when dissolved in water.
Has a pH level greater than 7.
Examples: Baking soda, antacids, caustic lime etc.
Indicators
Indicators are chemicals used to determine whether a substance is acidic or basic. They change color when added to a solution containing an acid or a base.
Types of Indicators
Natural Indicators: These are indicators derived from natural sources.
Litmus:
Litmus is a natural dye obtained from lichens.
It is commonly used in the form of litmus paper.
In water, litmus appears mauve (purple).
In acidic solutions, it turns red, while in basic solutions, it turns blue.
Turmeric:
Turmeric is a bright yellow powder derived from a plant, known as ‘Haldi’ in Hindi.
It contains a yellow dye and is used in the form of turmeric paper.
In basic solutions, turmeric turns red.
China Rose:
China rose, called ‘Gudhal’ in Hindi, is another natural indicator.
It is extracted from the red flowers of the China rose plant using water.
It changes color in response to acidity and basicity.
Man-Made Indicators: These indicators are chemically synthesized.
Phenolphthalein:
Phenolphthalein is colorless in acidic solutions and turns pink in basic solutions.
Methyl Orange:
Methyl orange is red in acidic solutions and yellow in neutral to basic solutions.
What is an olfactory indicator?
An olfactory indicator is a substance whose smell varies depending on whether it is mixed with an acidic or basic solution.
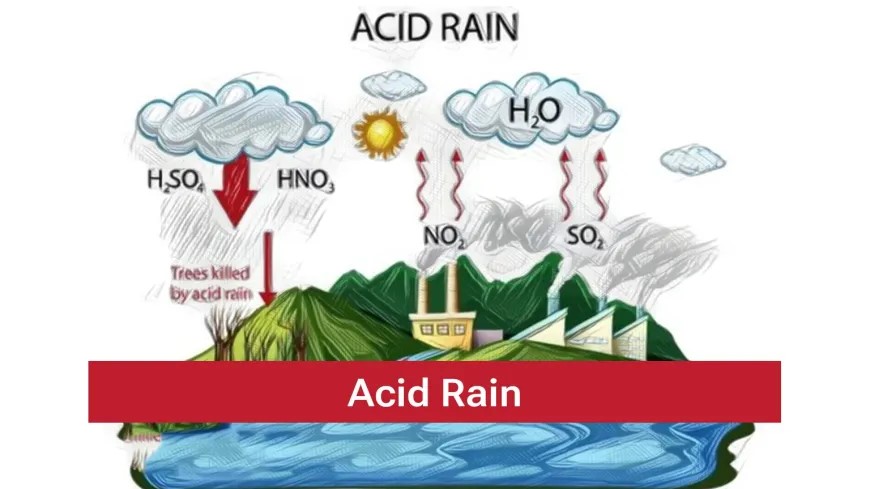
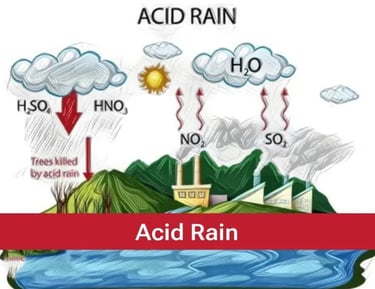
Acid Rain
The rain containing excess of acids called an acid rain. The rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide dissolve in rain drops to form carbonic acid, sulphuric acid and nitric acid respectively. It can cause damage to buildings, historical monuments, plants and animals.
Impact of Acid Rain
· Acid rain makes the water of lakes, ponds and rivers too acidic due to which fish and other aquatic animals get killed.
· Acid rain eats up the leaves of the trees gradually. By losing leaves, the trees die. Acid rain also damages crop plants in the fields.
· Acid rain damages the metal structures like steel bridges, etc when it falls on them.
· Acid rain damages the surfaces of buildings and monuments made up of marble.
Neutralisation
Acids and bases are chemically opposite substances. So, when an acid is mixed with a base, they neutralise (or cancel) the effect of each other. When an acid solution and a base solution are mixed in suitable amounts, both the acidic nature of the acid and the basic nature of the base are destroyed. The resulting solution is neither acidic nor basic. So, the reaction between an acid and base is known as neutralisation. In the process of neutralisation, salt and water are produced with the evolution of heat.
Indigestion
→ Milk of Magnesia (magnesium hydroxide) is an antacid used to neutralise the effect of the acid produced in our stomach.
Salts
A salt is formed when an acid and a base react.
The reaction in which acids react with bases resulting in the formation of salt and water are called neutralization reactions.
Types of salts:
A salt can be acidic basic or neutral.
Acidic salts: Acidic salt are formed when strong acids react with weak bases. These salts have a pH value of less than 7. For example, ammonium chloride (NH4ClNH4Cl), aluminium chloride (AlCl3AlCl3).
Basic salts: Basics salts are formed when strong bases react with weak acids. These salts have a pH value of more than 7. For example, sodium carbonate (Na2CO3Na2CO3), Sodium acetate (CH3COONaCH3COONa).
Neutral salts: Neutral salts are formed when strong acids react with strong bases. These salts have a pH value of 7. For example, sodium chloride (NaCl), Potassium Nitrate (KNO3).
Properties of Salts:
Most of the salts are soluble in water.
Solution of salts in water act as good conductor of electricity.
Some salts are white crystal whereas some are colored. For example, copper sulphate is blue in color and ferrous sulphate is green.
